Customer Success Story:
Exposing a Counterfeit Medical Shipment with a Murky Past
A shipment heading from India to the Middle East declared 130,200 units of medications on board.
Findings: Publican Detected a High Risk of Integrity Concern
Findings: Publican Detected High Risks to Public safety & Undervaluation
• Most of the world’s counterfeit medical supplies currently originate in India & China, raising a flag for the exporting country. (Counterfeit medications manufactured in sub-standard conditions may be faulty and pose a real danger to public health and safety.)
• The exporter—a pharmaceutical company—was found to have previous instances of fraudulent & corrupt practices, with recalls of medications imposed due to substandard manufacturing conditions.
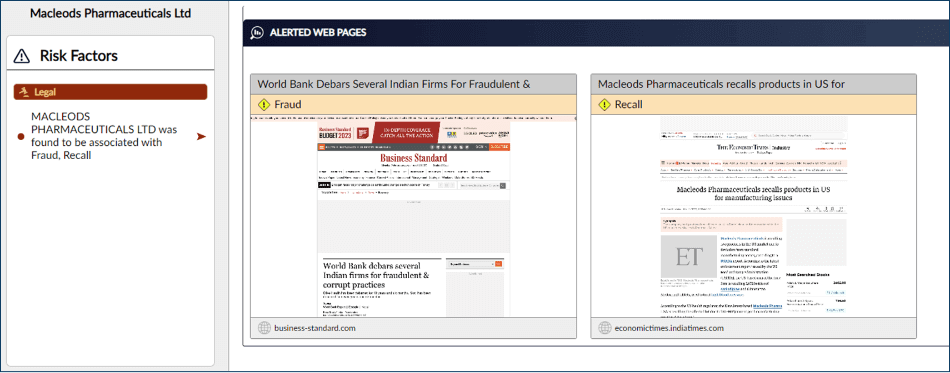
How did Publican uncover this?
By utilizing its unique wealth of sources to analyze every single facet of the exporter—including all previous reports, publication mentions, registrations, trade history, and every legal document connected to the pharmaceutical company in question—Publican concluded a high likelihood of integrity concern.
Impact
Not only was Publican’s system able to flag the shipment for customs inspection before arrival, but with the danger that counterfeits pose in an arena as precarious as medicine, it was able to provide an important safeguard for the nation’s public health.
Get in touch
-
21 Soho Square London W1D 3QP, UK



